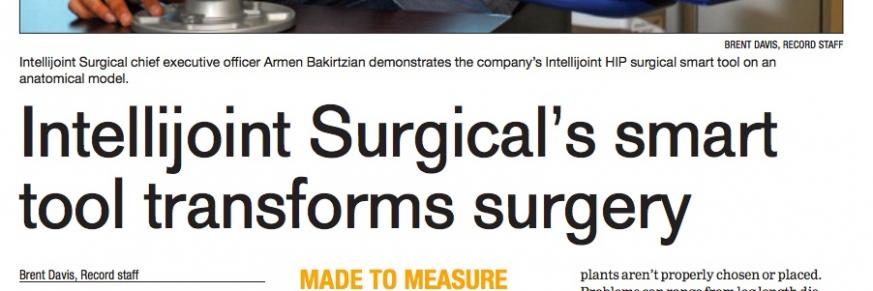
Local Orthopedic Surgeon, Ritesh Shah, MD, Receives Innovation Trailblazer Award for Adoption of New Navigation System for Hip Replacement Surgery
July 16, 2018 (Morton Grove, IL) – Orthopedic surgeon, Ritesh Shah, MD, was honored with the IJS Innovation Trailblazer Award of Excellence acknowledging early adoption of intellijoint HIP into his practice at Illinois Bone & Joint Institute and in an Ambulatory Surgery Center (ASC) setting at Illinois Sports Medicine & Orthopedic Surgery Center (ISMOSC) in Morton Grove. intellijoint HIP is a highly accurate 3D mini-optical navigation system […]